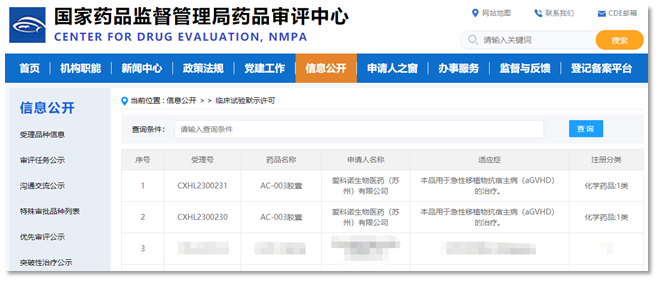
Suzhou China, May 4, 2023 —— Accro Bioscience (Suzhou) Limited announced today that its oral small-molecule RIPK1 inhibitor AC-003 has been approved by National Medical Products Administration (NMPA) to conduct clinical trials in China for the treatment of acute graft versus host disease (aGVHD). The purpose of this trial is to evaluate the safety, tolerability, and preliminary efficacy of AC-003 in aGVHD patients.
Acute graft versus host disease (aGVHD) is a common complication of allogeneic hematopoietic stem cell transplantation (alloHCT), with an incidence of approximately 30-50%, with 14-36% of patients developing severe aGVHD. aGVHD is the main cause of high mortality after stem cell transplantation, with only 25-30% of grade III aGVHD patients and 1-2% of grade IV aGVHD patients having a survival period greater than 2 years. Incidence rate of aGVHD in the United States, Europe and Japan: newly diagnosed aGVHD is 11500/year; Steroid refractory aGVHD is 5700/year. aGVHD mainly affects the skin (rash or dermatitis), liver (hepatitis or jaundice), and gastrointestinal tract (abdominal pain or diarrhea). The currently available therapies include corticosteroids add calcineurin inhibitors, JAK inhibitors, and other regimens with many side effects, including hyperglycemia, hypertension, bone necrosis, and increased risk of opportunistic infections.
About AC-003. According to preclinical research data from Accropeutics Bioscience, AC-003 maintains graft anti leukemia activity (GVL) with almost no inhibitory effect on hematopoietic cells and does not cause immune suppression. AC-003 effectively suppresses the progression of aGVHD in multiple animal models. Meanwhile, phase I clinical research data from China and the United States shows that AC-003 has good safety and tolerability. The mechanism of RIPK1 kinase function in aGVHD was published in Blood (https://doi.org/10.1182/blood.2022017262).
Dr. Xiaohu Zhang, Co-founder and CEO of Accropeutics Bioscience, said: “aGVHD is the second indication approved by NMPA to conduct clinical research after idiopathic pulmonary fibrosis (IPF) for AC-003. We will work diligently to move the program forward and hope AC-003 will one day benefiting patients worldwide.”
About Accropeutics Bioscience
Accro Bioscience (Suzhou) Limited is a clinical-stage biotech company with a core focus on molecular mechanisms of regulated cell death and related pathogenesis in human diseases. The Company has developed a robust portfolio with innovative compounds in various stages spanning from lead optimization to clinical trials. The company's RIPK1 inhibitor AC-003 was approved for clinical testing by FDA and NMPA in December 2021 and August 2022, respectively. AC-003 is nearing the end of Phase I clinical trials in the United States and China, and is about to start clinical trials for aGVHD patients. The company’s RIPK2 inhibitor AC-101 was approved by HREC in March 2023 for clinical testing and is in Phase I in Australia. In addition, AC-201, a selective TYK2/JAK1 inhibitor with huge potential for treating inflammatory and autoimmune diseases, has filed application for clinical testing in Australia. The company has multiple compounds in the PCC and preclinical research and development stages. Accropeutics Bioscience owns global rights of all its assets with more than 10 patents issued in China, Japan, Korea, US and EU.